Hyafilia preparations with hyaluronic acid.
Product Information Hyafilia Hiluronic Acid Fillers, Korea
HyaFilia fillers with hyaluronic acid, SNA MedtechCo. Ltd. (South Korea)
HyaFilia preparations are fillers of the 5th generation hybrid structure. They combined the best qualities of both mono and bi-phase products.
Compliance with the quality and safety of drugs international and Ukrainian standards proved by clinical trials, and officially confirmed by the relevant certificates.
CERTIFIED IN UKRAINE
The biphasic structure of the gel is a combination of a biotech stabilized hyaluronic acid of 85% and a non-stabilized fraction of 15%. The latest technology and composition provide the filler with high plasticity when injected. The implantation of the filler gives a fast and long-lasting effect not only due to the volumization, but also by improving the quality of the skin. The duration of the aesthetic effect is 6 - 14 months. HyaFilia preparations are biodegradable to water and carbon dioxide (H2O and CO2) and are completely eliminated from the body. This ensures the safety of the patient during the procedure of re-correction, and also allows the specialist to achieve the best result.
With natural biodegradation, there is no fragmentation and migration of the drug in the tissues, which is ensured by its high degree of thixotropy.
The drug is resistant to free radicals, which protects it from premature decay. The gel has a memory of a given shape, which is preserved after its introduction. This distinguishes HyaFilia from monophasic drugs. This property is the guarantor of the excellent effect of correction and the appearance of the patient.
The duration of the preservation of the effect depends individually on the age, somatic state of the body, its own enzymatic activity, lifestyle and on the characteristics of the drug.
HyaFilia – new generation of fillers based on hyaluronic acid. Their main distinguishing feature is the simultaneous combination of the advantages of monophasic and biphasic preparations. HyaFilia is recommended for the correction and smoothing of pronounced wrinkles, folds and post-acne scars, by introducing it directly into the dermis or subcutaneous tissue.
For more information, please contact us by phone.
Laboratory company SNA MedtechCo. Ltd. specializes in the development and production of non-animal hyaluronic acid-based biphasic fillers. High quality products, innovative developments have led to continuous growing demand from partners around the world.
The full line of Hyafilia fillers allows for a complete correction of wrinkles, folds, as well as to replenish the volume of tissue.

The production technology of HyaFilia fillers includes the process of stabilizing hyaluronic acid. This is due to the fact that the biodegradation of the native HA in a living organism occurs very quickly, and its reticulation with the help of a stabilizer can significantly increase the time of the splitting of HA by hyaluronidase. HyaFilia fillers produced by CHAMedtechCo.Ltd. Technology are stabilized with butanediol diglycidyl ether (BDDE).
The production technology of HyaFilia fillers allows to produce products with different properties for the correction of various aesthetic problems. Different use of HyaFilia preparations depends on the size of particles of reticulated hyaluronic acid and the degree of their crosslinking. HyaFilia Petit contains particles with the smallest size and the least degree of reticulation. In the filler, HyaFilia contains particles with an average size and average degree of reticulation. A drug HyaFilia Grand - with the largest particle size and a high degree of reticulation.
Characteristics of HyaFilia Hyaluronic Acid Fillers
The HyaFilia hyaluronic acid based filler line is characterized by a combination of effectiveness in correcting cosmetic defects, optimal plasticity when injected and laid out in tissues, complete biodegradation to water and carbon dioxide, the absence of a toxic component and minimal allergenicity.
Byphase by nature, Hyafilia fillers allow targeted and accurate face correction. Due to the addition of unstabilized hyaluronic acid, the preparations have sufficient plasticity as in monophasic fillers, which facilitates their distribution in the tissues. A high modulus of elasticity and a low modulus of fluidity ensure the effect is maintained (the duration of stay in tissues is 9-12 months). The natural, highly purified hyaluronic acid that makes up the fillers harmoniously corrects the age defects of the skin of the face, neck, décolleté, hands, prolonging the natural beauty and youth for a long time skin.
Hyaluronic acid, which is part of HyaFilia fillers
Origin: obtained by bacterial fermentation of an artificial strain of Streptococcus (Streptococcus equi), non-pathogenic for humans.
Benefits:
- Non-specific;
- biocompatible, non-immunogenic;
- non-animal origin (does not require allergic tests before the procedure);
- biodegradable;
- stable after administration;
- minimum residual hygroscopicity;
- crosslinked using BDDE.
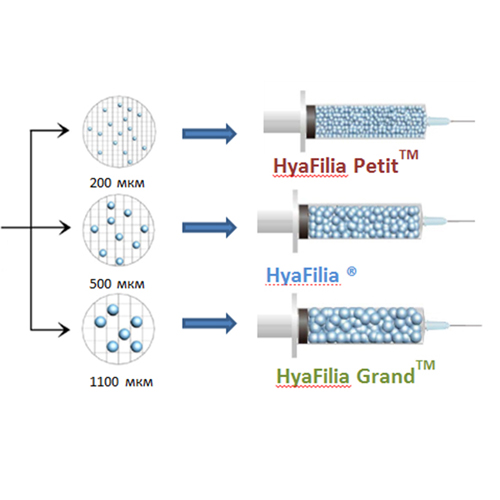
Hyaluronic Acid FILLER “HYAFILIA PETIT”
Hyafilia Petit - used to correct fine and superficial wrinkles.
Concentration: 20 mg / ml
Bulk ability: +++
Indications:
1. correction of nasolacrimal sulcus
2. fine lines wrinkles
3. “crow's feet”
4. perioral lines
5. linear wrinkles in the area between the eyebrows
6. lip contour correction
Guaranteed duration: 9 months.
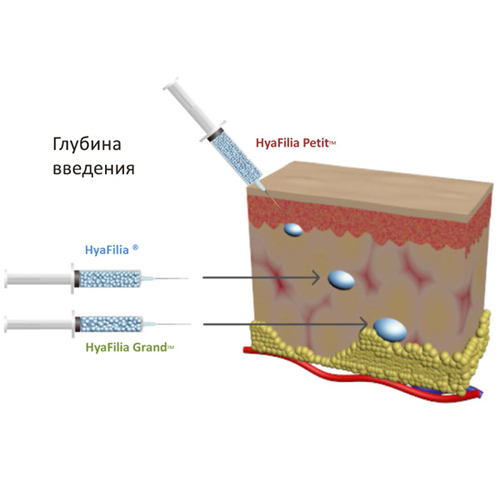
Injection level: deep dermis.
Hyaluronic Acid FILLER “HYAFILIA”
Hyafilia - is used for the correction of medium wrinkles, to increase the volume of the lips.
Concentration: 20 mg / ml
Bulk ability: ++++
Indications:
1. filling medium-depth wrinkles
2. correction of nasolabial folds
3. “puppet lines”
4. increase lip volume
5. perioral wrinkles
6. correction of hands
Guaranteed duration: 9 months.
Injection level: subdermal.
FILLER with hyaluronic acid “HYAFILIA GRAND”
Hyafilia Grand - the densest filler from the series to increase the volume of the cheekbones, chin, cheeks.
Concentration: 20 mg / ml
Bulk ability: +++++
Indications:
1. volumetric remodeling
2. increase in cheekbones, chin, temporal region
3. correction of the back of the nose
Guaranteed duration: up to 12 months.
Injection level: supraepaceous, deep
subdermal.
Benefits of HyaFilia
• A high degree of cross-linking means that the length of the polymer chains between the BDDE ester bonds is quite small. This greatly complicates the biodegradation of the filler with hyaluronidase and is clinically manifested in an increase in the duration of the aesthetic effect. Compared to other biphasic fillers, the degree of cross-linking of HyaFilia preparations is the highest - over 6 mol /%.
• A high concentration of reticulated hyaluronic acid causes a sufficient duration of filler degradation, and accordingly increases the duration of the correcting effect. Generally indicated is the total concentration of HA — both stabilized and native. But the native HA in the bi-phase filler is biodegraded quickly enough and its amount does not affect the duration of the filler effect. In HyaFilia preparations, the concentration of total HA is 20%, the concentration of crosslinked HA is 17%, this is the maximum concentration for modern biphasic fillers.
• The optimal ratio of viscosity and elasticity of HyaFilia preparations, with high plasticity, allows to achieve the most predictable result. In the filler HyaFilia Petit, designed to correct the least pronounced wrinkles, viscosity (55.5 Pa) and elasticity (295 Pa) are minimal. With a small viscosity (71.6 Pa) and a sufficiently high elasticity (357.8 Pa), HyaFilia is designed to correct changes in the average severity. HyaFilia Grand has the highest viscosity (164.6 Pa) and elasticity (472.2 Pa) and serves to correct the deepest wrinkles and folds.
• The minimum amount of residual protein in HyaFilia preparations, in comparison with other HA-based fillers, allows minimizing the risk of allergic reactions.
• The ratio of gel and liquid phases in HA-based fillers determines such properties of the filler as the plasticity of the preparation, the severity and duration of the effect. HyaFilia preparations are much more plastic than monophasic preparations due to the presence of a liquid component (15%). At the same time, the number of reticulated GK (85%) is significantly higher than that of similar biphasic preparations, which increases the period of biodegradation of HyaFilia fillers. The liquid component of the preparations consists not only of the native HA, but also of the modified non-reticulated HA, which also prolongs the correcting effect of the fillers.
• The absence of free (residual) BDDE in HyaFilia fillers has been convincingly proven by chromatography of preparations. On the HyaFilia chromatography charts, the signs of BDDE are not detected. This indicates that the entire BDDE is associated with GK and HyaFilia fillers do not have a toxic load on the patient’s body.
• The minimum content of endotoxins in HyaFilia preparations (less than 0.25 EU / ml) also indicates a high safety of preparations.
• A high degree of swelling (4 ml / g) leads to an increase in the effectiveness of HyaFilia compared to other biphasic fillers.
• The value of the osmolarity of HyaFilia preparations is as close as possible to the osmolarity of the inter-gland fluid of a healthy organism (300 mOsm / kg). Accordingly, the introduction of HyaFilia is more easily tolerated by the patient and is less pronounced signs of inflammation (redness and swelling) than the introduction of similar fillers.
Manufacturer: SNA MedtechCo. Ltd. specializes in the development and manufacture of bio-pharmaceutical products (cell therapy products, biological dosage forms and preparations based on recombinant protein), as well as cosmetics based on hyaluronic acid - the main raw material of bio-cosmetic products.
Founded in August 2013 SNA MedtechCo. Ltd., is a division of SNA Bio & Diostech, engaged in the sale and manufacture of medical devices and pharmaceutical products.
SNA MedtechCo. Ltd. develops and produces various growth factors and growth hormone preparations. The company conducts continuous research work in this direction, is engaged in the development of raw materials for the production of pharmaceutical products, as well as for the cultivation and differentiation of stem cell cultures.
SNA MeditechCo. Ltd. not only supplies raw materials for production, but also produces, supplies and improves the growth factor preparations needed to improve the quality of the skin, in collaboration with LG Household & Healthcare.
In addition, the company develops and supplies medical products based on hyaluronic acid.
Compliance with the production resources of SNA MeditechCo. Ltd. GMP and ISO standards, confirmation of product quality with KFDA and CE certificates and the availability of highly qualified personnel allowed the company to become one of the leading manufacturers of pharmaceutical and medical products. This is confirmed by the company's continuous success in the biomedical industry, manifested in the continuous expansion and development of the bio-product line.
BASIC ADVANTAGES BEFORE FAMOUS COMPETITORS:
• A high degree of cross-linking means that the length of the polymer chains between the BDDE ester bonds is quite small. This greatly complicates the biodegradation of the filler with hyaluronidase and is clinically manifested in an increase in the duration of the aesthetic effect. Compared to other biphasic fillers, the degree of cross-linking of HyaFilia preparations is the highest - over 6 mol /%.
• A high concentration of reticulated hyaluronic acid causes a sufficient duration of filler degradation, and accordingly increases the duration of the correcting effect. Generally indicated is the total concentration of HA — both stabilized and native. But the native HA in the bi-phase filler is biodegraded quickly enough and its amount does not affect the duration of the filler effect. In HyaFilia preparations, the concentration of total HA is 20%, the concentration of crosslinked HA is 17%, this is the maximum concentration for modern biphasic fillers.
• The optimal ratio of viscosity and elasticity of HyaFilia preparations, with high plasticity, allows to achieve the most predictable result. In the filler HyaFilia Petit, designed to correct the least pronounced wrinkles, viscosity (55.5 Pa) and elasticity (295 Pa) are minimal. With a small viscosity (71.6 Pa) and a sufficiently high elasticity (357.8 Pa), HyaFilia is designed to correct changes in the average severity. HyaFilia Grand has the highest viscosity (164.6 Pa) and elasticity (472.2 Pa) and serves to correct the deepest wrinkles and folds.
• The minimum amount of residual protein in HyaFilia preparations, compared with other HA-based fillers, allows you to minimize the risk of allergic reactions.
• The ratio of gel and liquid phases in HA-based fillers determines such properties of the filler as the plasticity of the preparation, the severity and duration of the effect. HyaFilia preparations are much more plastic than monophasic preparations due to the presence of a liquid component (15%). At the same time, the number of reticulated GK (85%) is significantly higher than that of similar biphasic preparations, which increases the period of biodegradation of HyaFilia fillers. The liquid component of the preparations consists not only of the native HA, but also of the modified non-reticulated HA, which also prolongs the correcting effect of the fillers.
• Lack of free (residual) BDDE in HyaFilia & n fillers
Tags: hyaluronic acid based fillers with hyaluronic acid, HyaFilia, SNA MedtechCo. Ltd., for wrinkle correction, for scar correction, for increasing volume